The C-cl Bond Dissociation Energy in Cf3cl
With increase in p character in an orbital bond length will increase while with increase in s character in an orbital bond length will decrease. The dissociation energy of the C-Cl bond decreases from 04 to 05 eV due to the water molecule.

Oneclass In Cf3cl The Caˆ Cl Bond Dissociation Energy Is 339 Kj Mol In Ccl4 The Ccl Bond Dissociat
Rotational centrifugal distortion and nuclear quadrupole 35 Cl and 37 Cl coupling constants have been.
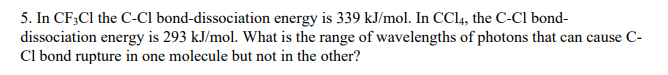
. What is the maximumwavelength of photons that can rupture this bondA. Answer 20 Watch For unlimited access to Homework Help a Homework subscription is. What is the maximum wavelength of photons that can rupture this bond.
Factors affecting bond energy. Concise Inorganic Chemistry for JEE by JD. According to a recommended classification scheme 3 this estimated Koc value suggests that chlorotrifluoromethane is expected to have moderate mobility in soil SRC.
1 f 0 at EN200 Td using the cross sections shown in Fig. 2 f 0 with d multiplied by 1. Sensitivity of the electron energy distribution function to variations in the dissociation cross section.
Up to 256 cash back In CF 3 Cl the CCl bond-dissociation energy is 339 kJmol. The maximum wavelengths of electromagnetic radiation required to rupture these bonds are ________ and ________ respectively asked Sep 12 2016 in Chemistry by Stanley1984 A 450 nm 307 nm B 742 nm 654 nm C 482 nm 248 nm D 353 nm 248 nm. Vertical ionization energies of the CF 3Cl molecule were derived tobe1246 and1310eVrespectivelyandtheverticalionization energies of the A2A 1B 2A 2C 2E and D2E states were 150 155 165 and 174 eV respectively81023 Once the internal energy exceeds the dissociation limits the CCl and CF bond cleavages of CF 3Cl may occur to produce.
What is the range of wavelengths of photons that can cause C- Cl bond rupture in one molecule but not in the other. The CCl bonddissociation energy in CF3Cl is 339 kJmol. A recent study on chlorotrifluoromethaneÁCO 2 evidenced that in the most stable structure the CO 2 moiety is almost parallel to the C 3v symmetry axis of CF 3 Cl with two fluorine atoms.
In CCl4 the C-Cl bond- dissociation energy is 293 kJmol. Bond energy is usually expressed in kJ mol 1. Full geometry optimizations have been carried out for S0 as well as relaxed potential energy calculations for both states along the C-Cl bond distance.
The CCl and CF bond dissociation energies in CF3Cl are 339 kJmol and 482 kJmol respectively. These calculations are typically accurate within k 3. See the answer Show transcribed image text Expert Answer Transcribed image text.
137 nmPlease explain indetails This problem has been solved. The CCl and CF bond dissociation energies in CF3Cl are 339 kJmol and 482 kJmol respectively. 353 nm 248 nm.
This set contains chlorinated organic molecules that consist of either sp3 - sp2 - or sp -hybridized CCl bonds. The species in this set have CCl BDEs at 298 K. The CO bond dissociation energy in CO2 is 799 kJmol.
The c-cl bond dissociation energy in cf3cl is 339 kjmol. Vertical excitation energies DeltaEvertical dissociation energies DeltaEdiss dissociation enthalpies DeltaHdiss and the oscillator strength f have also been computed. Greater is the bond energy stronger is the bond.
The dissociative photoionization of CF 3 Cl was investigated using threshold photoelectron photoion coincidence TPEPICO imaging in the energy range of 12301850 eV. Lee and Sudarshan Guha. What is the range of wavelengths of photons that can cause CCl bond rupture in one molecule but not in the other.
This problem has been solved. The rotational spectra of two isotopologues CF 335 ClCO and CF 337 ClCO of the CF 3 ClCO adduct have been investigated and analyzed using supersonic-jet Fourier transform microwave spectroscopy and found to have the features of a symmetric top. The coincident time-of-flight mass spectra and three-dimensional time-sliced images of the CF 2 Cl fragment were recorded at a few specific photon energies.
In CCl 4 the CCl bond-dissociation energy is 293 kJmol. The Koc of chlorotrifluoromethane is estimated as approximately 188 SRC using a measured log Kow of 165 1 and a regression-derived equation 2. Bond Energy Definition of Bond Energy The amount of energy required to break one mole of bonds of a particular type so as to separate them into gaseous atoms is called bond dissociation energy or simply bond energy.
For example d C C l in C H X 3 C l Å 178 Å d C C l in C F X 3 C l Å 175 Å. The ESD mass spectrum for CF3ClSi 111 77 of dose 13 1015 moleculescm2 in the range 10-71 amu at an incident electron energy of 250 eV was measured. In CF3Cl the C-Cl bond-dissociation energy is 339 kJmol.
PDF The dissociative photoionization of CF3Cl was investigated using threshold photoelectron photoion coincidence TPEPICO imaging in the energy. Department of Energys Office of Scientific and Technical Information Ab Initio Calculations and Three Different Applications of Unimolecular Rate Theory for the Dissociations of CCl4 CFCl3 CF2Cl2 and CF3Cl Journal Article OSTIGOV. To determine bond dissociation energies BDE heats of reaction and estimate reaction rate constants was obtained from ab initio calculations performed at the level of fourth-order Mgller- Plesset perturbation theory with empirical bond-additivity corrections referred to here as the BAC- MP4 method reported elsewhere 9.
The atmospheric photodissociation rate constants of. We herein report a dataset of 28 homolytic CCl bond dissociation energies BDEs to be known as the CCl28 dataset obtained using the benchmark-quality W1w thermochemical protocol. The maximum wavelengths of electromagnetic radiation required to rupture these bonds are ________ and ________ respectively.
Solved 5 In Cf3cl The C Cl Bond Dissociation Energy Is 339 Chegg Com
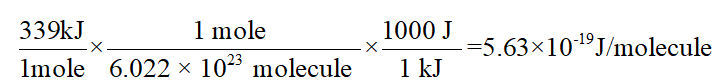
Answered In Cf3cl The C Cl Bond Di S Sociat Ion Bartleby
The C Cl Bond Distance In Ch3cl And Cf3cl Are 1 78a And 1 75a Respectively Comment On This Difference With The Help Of Bent S Rule Quora
Comments
Post a Comment